Biology (SJCR)
Stechnolock Journal of Case Reports
Full Text
Volume 1, Issue 1
Double Inlet Left Ventricle (DILV) with Mal-Posed Great Vessels: A Rare Complex Congenital Heart Disease
*Corresponding Author: Seyyed Hossein Hassanpour, Department of Medicinal Chemistry, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, Tel: +98 9171452844, E-mail: Dr.hossein1366@yahoo.com
doi: /sjcr.2021.1.107
Citation: SH Hassanpour, SZ Karami (2021) Double Inlet Left Ventricle (DILV) with Mal-Posed Great Vessels: A Rare Complex Congenital Heart Disease. Stechnolock J Case Rep 1: 1-5
Copyright: © 2021 SH Hassanpour. This is an open-access article distributed under the terms of Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Double inlet left ventricle (DILV) with transposition of the great vessels is rare in children with congenital heart disease. We present two cases. Baby ZK is a 28-day old female who presented with respiratory distress and cyanosis from the first day of life. Echocardiography showed a double inlet left ventricle and mal-posed great vessels. SH is a seven-month-old female who presented with breathlessness and cyanosis from the first day of life. Examination showed a soft pan-systolic murmur of grade 2 variety at the mid sternal boarder. ECG showed left ventricular dominance and abnormal T changes. Echocardiography revealed a double inlet left ventricle and mal-posed great vessels. Double inlet left ventricle and mal-posed great vessels is a rare complex cardiac anomaly of univentricular physiology. A high index of suspicion (especially if the new born presents with first day history of cyanosis and breathlessness) is necessary for quick referral and surgical intervention.
Keywords: Child; Double Inlet Left Ventricle and Mal-Posed Great Vessels; Echocardiography
Introduction
Double inlet left ventricle (DILV) with mal-posed great vessels is a rare congenital cardiac anomaly with seen in 0.05 to 0.1 per 1000 live births. [1] It is reported in 4% of neonates with congenital cardiac disease.2 Double inlet left ventricle (DILV) with mal-posed great vessels refers to a rare condition that constitutes 1.5% cases of congenital heart diseases, where a common ventricular chamber receives blood from two atria.[2] The most common type of this single ventricular physiology is the left ventricular type with variable great vessels connections, especially the malposed great vessels.[2] Symptoms vary from failure to thrive to breathlessness and cyanosis. Management is surgery. Children with a functionally single ventricle with unrestricted pulmonary blood flow will need a two staged palliation culminating in the Fontan circulation. [3] We present a case series involving two children with Double inlet left ventricle with transposed great arteries. This is the first time we are reporting this case in our setting. The case series will help paediatricians and cardiologist to always note that cyanosis from first day of birth should be accessed and investigated thoroughly to ensure early diagnosis of a possible cardiac anomaly and institute prompt treatment so as to avert death.
Case Presentation
In both cases, pregnancy had been largely uneventful and there was no positive history of miscarriages or congenital anomalies in previous pregnancies.
First Case
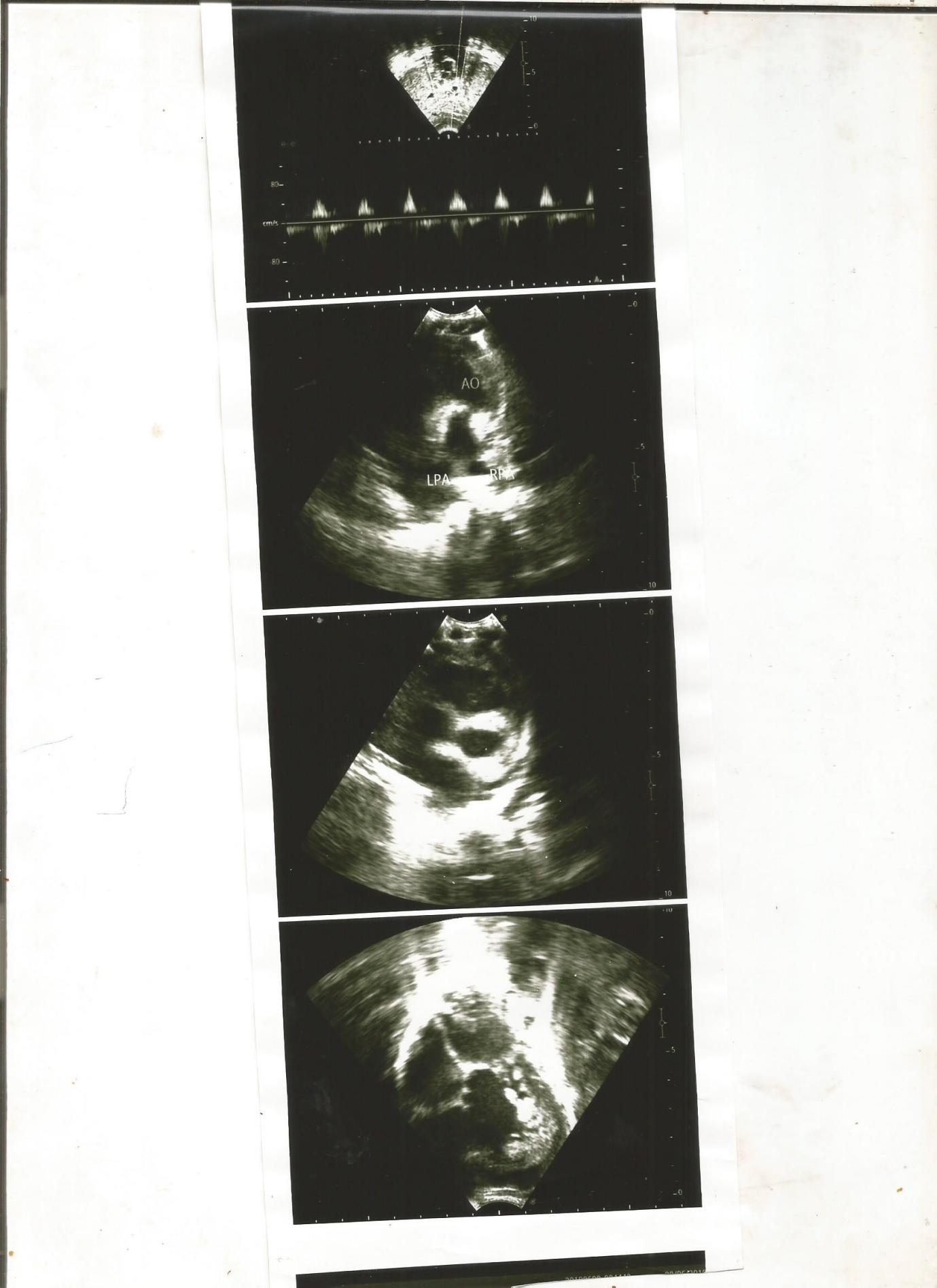
Baby ZK is a 28-day old female who presented with respiratory distress and cyanosis from the first day of life. Examination showed an ejection systolic murmur and splitting of second heart sound. Echocardiography revealed double inlet left ventricle and mal-posed great vessels. Child was commenced on anti-failure regimen and is being worked up for a –two- staged surgery (Glenn and Fortan) abroad.
Second Case
SH is a seven-month-old female who presented with breathlessness and cyanosis from the first day of life. Examination showed a soft pan-systolic murmur of grade 2 variety. Echocardiography showed a double inlet left ventricle and mal-posed great vessels. Electrocardiogram showed left ventricular dominance and abnormal T changes.
Discussion
Double inlet left ventricle and mal-posed great vessels (DILV) is a functionally uni-ventricular heart with a well-defined atrioventricular connection in which the morphologically left ventricle receives more than 50% of the atrioventricular valves when they are separated, or more than 75% of the common atrioventricular valve. [3] In this complex cardiac anomaly, both atria are connected to the left ventricle. Usually there is a hypoplastic right ventricle (RV), which may be on the opposite side of the heart mimicking "Corrected Transposition". The arteries usually arise with the aorta from the RV and the pulmonary artery from the LV (Transposition). Our index patient presented with echocardiographic features of a both atria supplying blood to a single ventricle with a malposed vessel but restricted pulmonary blood flow. Death in most cases ensues primarily from congestive cardiac failure, arrhythmias or sudden death if surgery is not done. [4] Both of our patients were clinically stable possibly due to increased pulmonary pressures. The female preponderance seen in our study was also reported by Alsoufi et al [5] who noted a female preponderance in their series. This did not agree with the fact that heart lesion from cono-truncal abnormalities is usually commoner in males. [4] There are associated cardiac lesions in children with DILV. These include; coarctation of the aorta, pulmonary atresia, and pulmonary valve stenosis. [4] Our first case presented with valvar pulmonary stenosis. However, Infants with DILV who have no pulmonary stenosis usually develop congestive heart failure within the first few weeks of life due to the increased blood flow and need pulmonary arterial banding early in the neonatal period. In situations of pulmonary outflow obstruction, a systemic-to-pulmonary artery shunt is required to provide adequate blood flow for oxygenation. [6] The clinical symptoms include failure to gain weight, breathlessness, poor feeding, cyanosis and heart murmur. Our first case presented with a loud pulmonary component of the second heart sound (P2) because of the anteriorly placed aorta and an ejection systolic murmur from pulmonary stenosis. We commenced anti-failure regimen for our cases and they are currently being worked up for surgery outside the country since we do not have the facilities for the two-staged surgery. Management usually involves staged surgery culminating with Fontan palliation. A shunt-dependent circulation and pulmonary artery-aortic anastomosis can also be done as a palliative surgical intervention (Damus-Kaye-Stansel connection). Here, the main pulmonary artery is divided and the proximal main pulmonary artery stump is then implanted into the side of the aorta. [6] Besides, with advances in non-invasive investigations, surgical and postoperative care outcomes have improved tremendously in the last few years. [7, 8] After the Fontan operation, most children with DILV can be enrolled in school and could be gainfully employed later in life. [7,8] However, some children still present with complications such as arrhythmias, atrioventricular valve insufficiency, ventricular failure, subaortic obstruction, plastic bronchitis and protein-losing enteropathy post-surgery. [7-12] The management of DILV and other complex cardiac disease is a very big challenge in developing countries. There is an urgent need for capacity building and training and retraining of personnel in order to manage DILV and other complex congenital heart diseases. [13-16] Ordinarily DILV is uncommon, but the presence of DILV with associated mal-position of great vessels, makes it one of its kind in our setting till date.
Conclusion
Double inlet left ventricle and mal-posed great vessels is a rare complex cardiac heart anomaly of uni-ventricular physiology. A high index of suspicion (especially if the new born presents with first day history of cyanosis and breathless) is necessary for quick referral and surgical intervention.
Competing interests
The authors declare that there is no conflict of interest regarding this study.
Patient's Consent
Informed consent was obtained from the parents of the children.
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2016) Heart disease and stroke statistic - 2016 Update: A Report from the American Heart Association. Circulation 133: e38-360.
- Checchia PA, McGuire JK, Morrow S, Daher N, Huddleston C, et al. (2006) A risk assessment scoring system predicts survival following the Norwood procedure Pediatr Cardiol 27: 62-6.
- Meyer SL, Jongbloed M, Ho SY, Bartelings MM, McCarthy KP, et al. (2017) Intracardiac anatomical relationships and potential for streaming in double inlet left ventricles. PLoS One 12: e0188048.
- Lahiri S, Gil W, Daria S, Joshua G, Parul J, et al. (2020) Genetic abnormalities/syndromes significantly impact perioperative outcomes of conotruncal heart defects. Ann Pediatr Cardiol 13: 38-45.
- Alsoufi B, McCracken C, Kanter K, Shashidharan S, Kogon B (2017) Current Results of Single Ventricle Palliation of Patients with Double Inlet Left Ventricle. Ann Thorac Surg 104: 2064-71.
- Gidvani M, Ramin K, Gessford E, Aguilera M, Giacobbe L, (2011) Prenatal diagnosis and outcome of fetuses with double-inlet left ventricle. AJP Reports 1: 123-8.
- Cook AC, Anderson RH (2006) The anatomy of hearts with double inlet ventricle. Cardiology in the Young. Cambridge University Press 16: 22–6.
- Hagler DJ, O’Leary PW (2000) Cardiac malpositions and abnormalities of atrial and visceral situs. In Moss and Adams Heart Disease in Infants, Children and Adolescents, edn 6. Edited by Allen HD, et al. Philadelphia: Lippincott Williams and Wilkins 1129-50.
- Vyas H, Hagler DJ (2007) Double inlet left ventricle. Curr Treat Options Cardiovasc Med 9: 391–8.
- Margossian RE, Solowiejczyk D, Bourlon F, Apfel H, Gersony WM, et al. (2002) Septation of the single ventricle: revisited. J Thorac Cardiovasc Surg 124: 442–7.
- Earing MG, Cetta F, Driscoll DJ, Mair DD, Hodge DO, et al. (2015) Long-term results of the fontan operation for double-inlet left ventricle. Am J Cardio l 96: 291–8.
- Mair DD, Hagler DJ, Julsrud PR, Puga FJ, Schaff HV, et al. (1991) Early and late results of the modified Fontan procedure for double-inlet left ventricle: the Mayo Clinic experience. J Am Coll Cardiol 18: 1727–32
- Pfitzer C, Helm PC, Ferentzi H, Rosenthal LM, Bauer UMM, et al. (2017) Changing prevalence of severe congenital heart disease results from the National Registry for Congenital heart disease in Germany. Congenit Heart Dis 12: 787-93.
- Nwafor IA, Novick W, Adiele DK, Eze JC, Ezemba N, et al. (2016) Repair of Truncus Arteriosus, Type I in Nigeria. A Case Report. J Vasc Med Surg 4: 1- 3.
- Duru CO, Mesiobi-Anene N, Ujuanbi S, Akalonu E, Aliyu I, et al. (2018) Pattern and outcome of patients referred abroad for cardiac surgery from a tertiary centre in the Niger Delta region of Nigeria. Nig J Card 15: 9-13.
- Ujunwa FA, Ujuanbi IS, Chinawa JM (2021) Complex congenital heart diseases among children presenting for cardiac surgery in a tertiary health facility in Enugu; South-East Nigeria. A rising trend. Niger J Clin Pract 24: 100-3.